
Greetings everyone,
In this topic, we will delve into the intricacies of protein structure. Before we immerse ourselves in this subject, have you ever considered that the growth of animals and humans depends on the nature of their diet? Undoubtedly, you have. Maybe you're observing the link between this fact and the structure of proteins. Therefore, let's collectively view the aforementioned video.
Within this concise one-minute video, you will see the important of protein structure in your life. You will also discover that complex protein can be obtained from your daily diet. Upon completing this video, you will get an idea on how this topic align with your daily life, enhancing your ability to apply the acquired knowledge more effectively.
Happy exploration !!!
TITLE
Three-Dimensional Structure of Protein
INTRODUCTION
In this topic, you will learn about the three-dimensional structure of proteins, also known as their tertiary structure, which is a remarkable and crucial aspect of biochemistry. Proteins, which are essential molecules in living organisms, carry out a wide array of vital functions, from enzymatic reactions to structural support. The precise folding and arrangement of atoms in a protein determine its unique three-dimensional shape, and this shape is intricately linked to its function. The structure of a protein is the key to understanding how it interacts with other molecules and performs its biological role. The folding process is guided by the sequence of amino acids in the protein's primary structure and influenced by various forces, including hydrogen bonds, disulfide bridges, and hydrophobic interactions. A detailed exploration of the three-dimensional structure of proteins is essential for unlocking the secrets of biology and biochemistry.
TOPICAL LEARNING OUTCOMES
At the end of this learning process, student should be able to:
Techniques in protein structure determination (TLO3)
To understand about the topic 3D structure of protein, you should refer to the video and guide below:
In the above video, we're diving into the fundamental aspects of protein, focusing specifically on the structure and function. Just like the letters of the alphabet form words, amino acids come together to create the intricate language of proteins. I'm excited to guide you through this fascinating story.
Proteins, roles and important
We'll begin by exploring what proteins are and their roles. From the 22nd to the 50th second, you were introduced to the concept that proteins are macromolecules composed of amino acids, playing crucial roles in tissue structure, enzyme functions, and hormone regulation. Between 2:31 and 3:05, you were informed about the importance of proper protein folding in maintaining life.
Primary Structure and Secondary Structure (TLO1)
Next, from 51 seconds to 1 minute and 14 seconds, you were briefed on the primary structure, which is the linear arrangement of amino acids in a protein chain. This basic sequence serves as the blueprint for a protein's function, akin to the order of colorful beads on a string.
From there, you ventured into the realm of secondary structure (1:15 to 1:45). Imagine this as the folding and bending of the protein chain into recognizable patterns, such as helices (like metal springs) and beta-sheets (like folded paper). These secondary structures add depth and complexity to the protein, influencing how it interacts with its surroundings, particularly through hydrogen bonds.
Primary Structure & Secondary Structure
Based on the provided learning material, you are required to complete self learning activity and self assessment of TLO1
Imagine proteins as molecular architects, meticulously folding and arranging their structures to perform precise functions within living organisms. Up to this point in the video, we have explored the blueprint of proteins (primary structure) and the repeating patterns that emerge (secondary structure). Now, let's delve into the next level of complexity — the three-dimensional masterpiece known as tertiary structure (1:46 to 2:06).
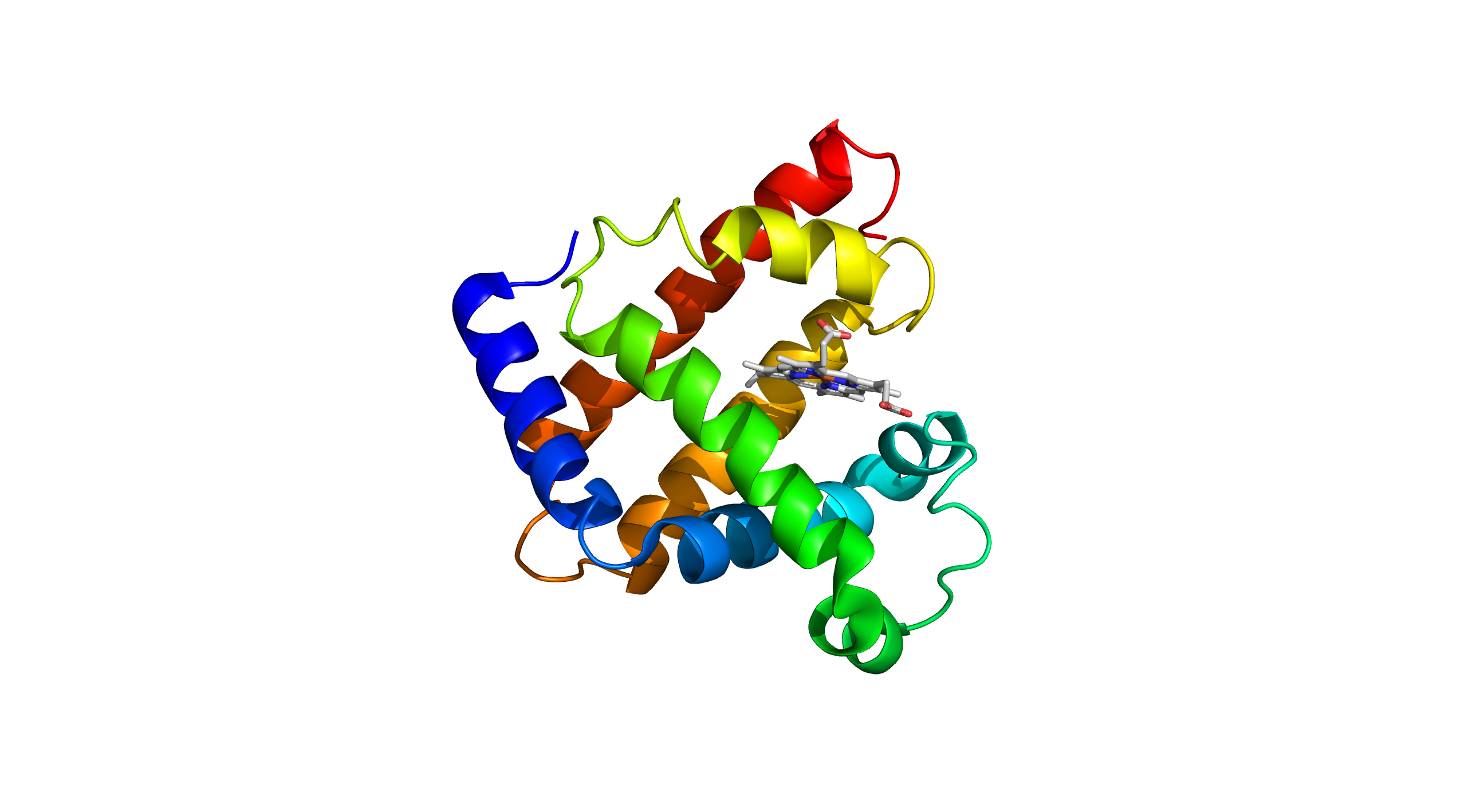
Just as an artist meticulously sculpts a statue, the tertiary structure dictates the precise arrangement of a protein's atoms in space, giving it a unique and functional form. The tertiary structure of a protein refers to the overall three-dimensional shape formed by the folding and twisting of its polypeptide chain. This complex shape is stabilised by various interactions, including hydrogen bonds, ionic bonds, disulphide bridges, and hydrophobic interactions. Myoglobin, a well-known example of a protein with a distinct tertiary structure, is responsible for oxygen storage in muscle tissues. It is composed of a single polypeptide chain that folds into a globular shape, creating a hydrophobic pocket where the heme group binds. The heme group, containing an iron atom, is crucial for myoglobin's ability to bind and release oxygen molecules efficiently. Myoglobin's tertiary structure is essential for its function, as it allows the protein to maintain its stability and effectively perform its role in oxygen transport and storage within muscle cells.
Tertiary Structure (PDB ID: 3RGK)
You have explored the intricacies of primary and secondary structures, uncovering the linear sequences and recurring patterns that form the backbone of proteins. Now, let's move to another level of complexity to understand how individual protein subunits come together in a beautifully orchestrated dance — the quaternary structure (2:06 to 2:31).
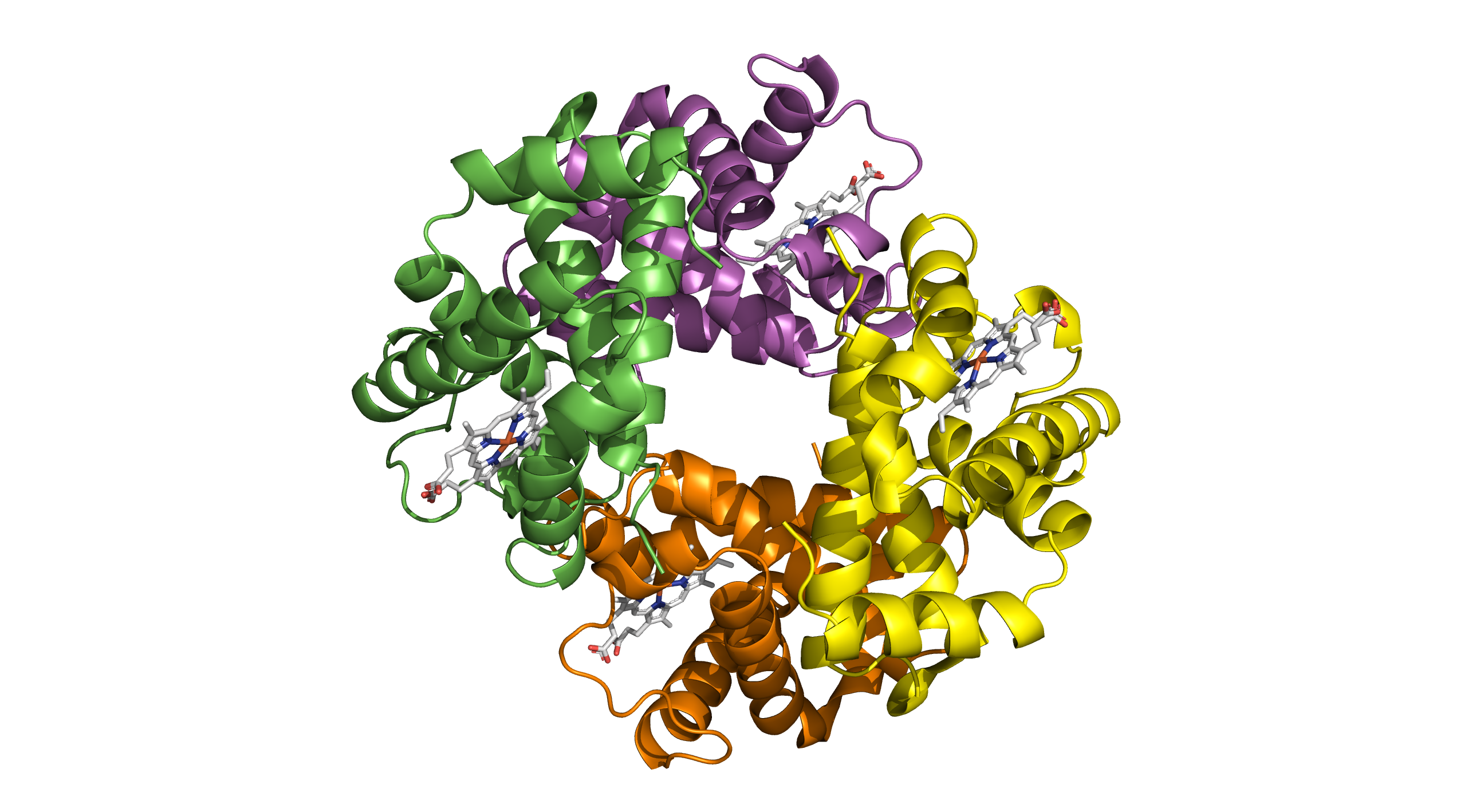
Imagine a biological symphony where multiple players join forces, each contributing its unique melody to create a harmonious piece. This is the essence of quaternary structure – the arrangement and interaction of multiple protein subunits to form a functional, dynamic whole.
Quaternary structure is the highest level of protein structure, characterised by the assembly of multiple polypeptide chains into a single, functional unit. In the case of haemoglobin, this quaternary structure is essential for its function. Haemoglobin is composed of four polypeptide chains: two alpha chains and two beta chains. Each of these chains contains a heme group that can bind to oxygen. The quaternary structure of haemoglobin allows it to efficiently transport oxygen from the lungs to tissues throughout the body and return carbon dioxide back to the lungs for exhalation. The cooperative binding of oxygen, where the binding of one oxygen molecule increases the affinity of the remaining sites for oxygen, is a direct result of its quaternary structure. This structural configuration is crucial for the physiological role of haemoglobin in maintaining proper oxygen levels in the bloodstream.
Quaternary Structure (PDB ID: 4N7N)
Based on the provided learning material, you are required to complete self learning activity and self assessment of TLO2
Protein structure determination techniques (TLO3)
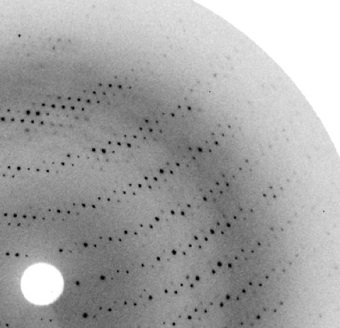
There are three common techniques used by researchers to determine the structure of protein (3:06 to 3:55). X-ray crystallography is a powerful and widely used technique for determining the atomic and molecular structure of proteins. By directing X-rays at a crystallized sample of the protein, the X-rays are diffracted in various directions. The resulting diffraction pattern can be analyzed to deduce the electron density within the crystal. Scientists then use this electron density map to build a detailed model of the protein's three-dimensional structure. This method has been pivotal in understanding the structural basis of protein function, aiding in drug design, and unraveling the complexities of biological processes at the molecular level. The precision of X-ray crystallography has made it an invaluable tool in structural biology and biochemistry.
 Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to determine the structure of proteins at the atomic level. Unlike X-ray crystallography, which requires protein crystallization, NMR can analyze proteins in solution, closely mimicking their natural environment. This method exploits the magnetic properties of certain atomic nuclei, primarily hydrogen (1H), carbon (13C), and nitrogen (15N), to provide detailed information about the protein's three-dimensional structure, dynamics, and interactions. By applying a strong magnetic field and radiofrequency pulses, NMR measures the resonance frequencies of these nuclei to deduce the distances between atoms and the angles of chemical bonds. This data is then used to construct a detailed model of the protein, revealing insights into its function and interactions with other molecules. NMR spectroscopy is particularly valuable for studying small to medium-sized proteins and offers unique advantages in understanding the conformational flexibility and dynamics that are crucial for many biological processes.
Nuclear Magnetic Resonance (NMR) spectroscopy is a powerful analytical technique used to determine the structure of proteins at the atomic level. Unlike X-ray crystallography, which requires protein crystallization, NMR can analyze proteins in solution, closely mimicking their natural environment. This method exploits the magnetic properties of certain atomic nuclei, primarily hydrogen (1H), carbon (13C), and nitrogen (15N), to provide detailed information about the protein's three-dimensional structure, dynamics, and interactions. By applying a strong magnetic field and radiofrequency pulses, NMR measures the resonance frequencies of these nuclei to deduce the distances between atoms and the angles of chemical bonds. This data is then used to construct a detailed model of the protein, revealing insights into its function and interactions with other molecules. NMR spectroscopy is particularly valuable for studying small to medium-sized proteins and offers unique advantages in understanding the conformational flexibility and dynamics that are crucial for many biological processes.
Cryo-electron microscopy (cryo-EM) has revolutionized the field of structural biology by providing unprecedented insights into the architecture of proteins at near-atomic resolution. This technique involves rapidly freezing protein samples to preserve their native structures and then imaging them using an electron microscope. Unlike traditional X-ray crystallography, which requires proteins to form crystals, cryo-EM can study proteins in their natural, non-crystalline states. This makes it particularly valuable for examining large, complex proteins and dynamic assemblies that are difficult to crystallize. With advancements in detector technology and image processing algorithms, cryo-EM has enabled researchers to visualize intricate details of protein structures, leading to a deeper understanding of their functions and interactions, and opening new avenues for drug discovery and design.
Based on the provided learning material, you are required to complete self learning activity and self assessment of TLO3
Based on the learning materials provided, you are requested to:
Dear students,
After downloading a protein PDB from the RCSB website, discuss its primary and secondary structures. Compare the helices and beta-pleated sheets.
Dear students,
Please draw both structures and then compare the functions of myoglobin and haemoglobin in a document.
Then, scan the document as a single PNG file and upload it to the Lightbox gallery link.
Dear students,
List the advantages and disadvantages of X-ray crystallography, NMR spectroscopy, and Cryo-EM. Provide the answers in table form for easy comparison.
In order to test either you able to describe the primary and secondary structure, compare between tertiary and quaternary structure, and discuss the techniques used in protein structure determination, please assess the quizzes below:
Dear Students,
Get ready for an exciting opportunity to challenge your knowledge and enhance your learning experience! This quiz is a great way to assess your understanding of the course material and have some educational fun.
Why Join the Quiz
How to Participate:
Let's make this quiz an engaging and educational experience for all.
We look forward to seeing your active participation!
To obtain more info regarding the topic , you are encouraged to refer the following sources:
1. Youtube videos:
2. Youtube videos:
3. Research Article:
The below pdf file describes about the Multiscale MD simulation of wild-type and sickle hemoglobin aggregation published from Wiley Publisher
4. Youtube videos:
The three-dimensional (3D) structure of proteins is a fundamental aspect of biochemistry and molecular biology. Proteins, essential biomolecules in living organisms, adopt specific 3D shapes that are intricately linked to their functions. This structure is organized into primary, secondary, tertiary, and, in some cases, quaternary levels. The primary structure represents the linear sequence of amino acids, while the secondary structure involves local folding patterns, such as alpha helices and beta sheets, held together by hydrogen bonds. The tertiary structure defines the overall 3D shape of the protein, determined by various forces like disulfide bridges and hydrophobic interactions. Some proteins consist of multiple subunits with quaternary structure. The 3D structure is crucial for understanding how proteins interact with other molecules and carry out their biological roles, making it a central topic in the study of biochemistry and molecular biology. Exploring protein structures through molecular modeling and visualization offers a practical and engaging way to gain insights into their complex architecture and functions.
Below are the important key terms in learning of topic 3D Structure of Protein: